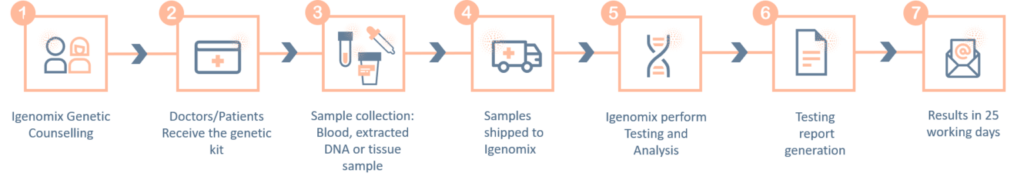
Panel de precisión de ventriculomegalia
Ventriculomegalia es el término utilizado para describir la dilatación ventricular no relacionada con el aumento de la presión del líquido cefalorraquídeo, como la dilatación debida a disgenesia o atrofia cerebral.